Background:
Cardiac involvement by light chain amyloidosis (AL) is generally associated with an unfavorable outcome. Bortezomib-based induction, and high-dose melphalan followed by autologous hematopoietic stem cell transplantation (auto-HCT) in eligible patients is associated with best long-term outcomes. We report the outcome of cardiac AL patients who underwent auto-HCT at our institution.
Methods:
We retrospectively reviewed all patients with cardiac AL who received auto-HCT between January 1997 and December 2018 at our institution. Hematologic and cardiac organ responses were evaluated according to the Consensus Guidelines for AL (R Comenzo et al. Leukemia 2012). Revised Mayo staging system was used for cardiac staging (S Kumar et al. JCO 2012). Progression free survival (PFS) and overall survival (OS) were calculated from the date of transplant. Survival was estimated using Kaplan Meier method and compared using log rank test. Cox proportional hazard models were used for adjusted survival analysis.
Results:
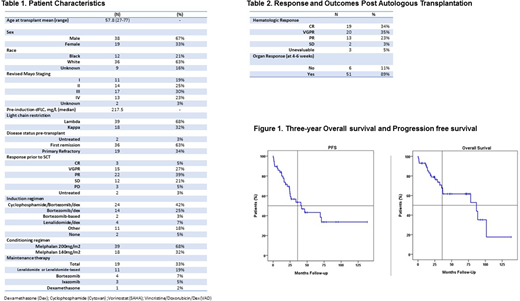
57 patients were identified and baseline characteristics summarized in Table 1. Thirty eight patients (67%) at diagnosis and 17 (30%) at auto-HCT were evaluable by the revised Mayo staging system. Eleven (19%), 14 (25%), 17 (30%), and 13 (23%) patients had stage 1, 2, 3 and 4 disease, respectively, while the stage was unknown in 2 (3%) patients. Twenty-four (42%) patients received induction with a combination of cyclophosphamide, bortezomib, and dexamethasone (CyBorD), 14 (25%) received bortezomib and dexamethasone, and 2 (3%) received other bortezomib-based induction (Table 1). Based on hematologic response criteria, 3 (5%), 15 (27%) and 22 (39%) patients achieved complete response (CR), a very good partial response (VGPR), or partial response (PR) to induction, with an overall response rate (ORR) of 71%. All patients underwent peripheral blood stem cell (PBSC) mobilization with filgrastim, with or without plerixafor. Thirty-nine (68%) patients received melphalan 200mg/m2 and 18 (32%) received melphalan 140mg/m2 as preparative regimen. Nineteen patients (33%) received maintenance therapy post auto-HCT. One-hundred day and 1-year post auto-HCT non-relapse mortality rate was 5% (3 patients). Best post auto-HCT hematologic ORR was 92%, with 19 (34%), 20 (35%), and 13 (23%) patients achieving CR, VGPR and PR, respectively. Based on the consensus guidelines for cardiac response in AL using NT-proBNP or NYHA class, 51 patients (89%) had a cardiac organ response at their last evaluation (Table 2). Median follow up in surviving patients was 32.9 months (range 5.1 - 140.6). The 3-year PFS was 53.5% [95% CI 38.6-68.4%], and 3-year OS was 67.8% [53.9-81.7%]. On univariate analysis, melphalan 200 vs. 140 (p=0.017, HR 0.387 95%CI 0.178- 0.844) was associated with a better PFS, but none of the variables had an impact on PFS or OS on a multivariate Cox regression analysis, perhaps due to a small sample size.
Conclusion:
In this retrospective analysis we showed that in transplant-eligible patients with advanced cardiac AL, high-dose melphalan and auto-HCT is associated with a low (5%) NRM, an organ response rate of almost 90%, and a 3-year OS of almost 70%.
Bashir:Takeda: Other: Advisory Board, Research Funding; Acrotech: Research Funding; Celgene: Research Funding; StemLine: Research Funding; KITE: Other: Advisory Board; Purdue: Other: Advisory Board; Amgen: Other: Advisory Board. Nieto:Secura Bio: Other: Grant Support; Astra Zeneca: Other: Grant Support; Novartis: Other: Grant Support; Affimed: Consultancy, Other: Grant Support. Hosing:NKARTA Inc.: Consultancy. Popat:Bayer: Research Funding; Novartis: Research Funding. Lee:Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy; Genentech: Consultancy; Daiichi Sankyo: Research Funding; Sanofi: Consultancy; Regeneron: Research Funding. Patel:Takeda: Consultancy, Research Funding; Precision Biosciences: Research Funding; Oncopeptides: Consultancy; Poseida: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Consultancy, Research Funding; Cellectis: Research Funding; Celgene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Manasanch:Merck: Research Funding; Sanofi: Honoraria; GSK: Honoraria; Takeda: Honoraria; Quest Diagnostics: Research Funding; Adaptive Biotechnologies: Honoraria; JW Pharma: Research Funding; Novartis: Research Funding; BMS: Honoraria; Sanofi: Research Funding. Thomas:X4 Pharma: Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Research Funding; Xencor: Research Funding; Pharmacyclics: Other: Advisory Boards. Kaufman:Karyopharm: Honoraria; Janssen: Research Funding; Bristol Myers Squibb: Research Funding. Orlowski:Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; STATinMED Research: Consultancy; Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties. Champlin:Takeda: Patents & Royalties; Genzyme: Speakers Bureau; DKMS America: Membership on an entity's Board of Directors or advisory committees; Cytonus: Consultancy; Omeros: Consultancy; Johnson and Johnson: Consultancy; Actinium: Consultancy. Qazilbash:Bioclinica: Consultancy; Amgen: Research Funding; Angiocrine: Research Funding; Bioline: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal